Could Tiny Blood Clots Cause Long COVID’s Puzzling Symptoms?
Several experts believe that micro-clots may be a contributing factor to long COVID. But could tiny blood clots really be the cause for long COVID and its puzzling symptoms?
When Lara Hawthorne, an illustrator in Bristol, UK, started experiencing peculiar symptoms after getting COVID-19, she believed it was not because to the virus. Her initial ailment had been minor. “I’ve been triple vaccinated. I felt quite protected ,” she recalls. She was still sick months later, with a range of often debilitating symptoms, including earaches, tinnitus, congestion, migraines, vertigo, heart palpitations, muscle soreness, and more. Hawthorne was so weak on certain days that she could not get out of bed. When she eventually saw her doctor, the diagnosis was exactly what she had feared: long COVID.
She grew increasingly desperate as she failed to find relief. After reading an opinion post in The Guardian regarding blood clots being to blame for chronic COVID symptoms, Hawthorne contacted a physician in Germany who is treating patients with blood thinners and a blood filtering technique. She has not heard back yet — rumor has it that individuals are kept on the waiting list for months — but if she has the chance to go there for these experimental treatments, she would. “I do not want to put my health on hold when I am feeling so bad,” she says.
Long COVID has perplexed researchers: hundreds of experiments have attempted to unravel its mechanism, with little success. Now, many scientists and a growing number of patients suffering from the ailment are rallying around the untested notion that microscopic, persistent clots are restricting blood flow to crucial organs, resulting in the unusual constellation of symptoms that people experience.
Proponents of the notion comprise Etheresia Pretorius, a physiologist at Stellenbosch University in South Africa, and Douglas Kell, a systems biologist at the University of Liverpool in the United Kingdom, who headed the first team to visualize micro-clots in the blood of persons with long COVID. They claim that the data implicating micro-clots is incontrovertible, and they demand trials of the anticoagulant treatments being explored by Hawthorne. Pretorius wrote the Guardian piece that piqued Hawthorne’s interest.
Several haematologists and COVID-19 researchers, however, are concerned that the enthusiasm for the clot hypothesis has outrun the data. They want larger studies and more conclusive evidence. They are also concerned about people pursuing unproven, potentially dangerous treatments.
When it pertains to long COVID, “we’ve now got little scattered of bits of evidence,” says Danny Altmann, an immunologist at Imperial College London. “We’re all scuttling to try and put it together in some kind of consensus. We’re so far away from that. It’s very unsatisfying.”
Cascade of clots
Pretorius and Kell first met over a decade ago. Pretorius had been researching the role of iron in clotting and had forgotten to cite some of Kell’s work. They started talking when he reached out. “We had a Skype meeting and then we decided to work together,” Pretorius explains. They discovered strange, thick clots that resist breaking down for years in persons suffering from a variety of ailments. The study led them to the conclusion that certain substances, such as iron, proteins, or bacterial cell wall fragments, may cause these aberrant clots.
Blood clotting is a complicated process, but one of the major participants is fibrinogen, a cigar-shaped, soluble protein that circulates freely in the bloodstream. When a cell is injured, the enzyme thrombin is released, which converts fibrinogen into an insoluble protein called fibrin. Strands of fibrin loop and crisscross, forming a web that aids in the formation of a clot and the cessation of bleeding.
Under a microscope, this web looks like “a nice plate of spaghetti,” according to Kell. However, the clots detected by the investigators in a variety of inflammatory disorders appear to be distinct. They are “horrible, gunky, dark,” according to Kell, “such as you might get if you half-boiled the spaghetti and let it all stick together.” According to Kell, Pretorius, and their colleagues, the fibrin has misfolded1, resulting in a gluey, ‘amyloid’ form of itself. According to Kell, it does not take much misfolding to spawn tragedy. “If the first one changes its conformation, all the others have to follow suit,” similar to prions, the contagious misfolded proteins that cause diseases like Creutzfeldt-Jakob disease.
Pretorius originally noticed these odd, thickly matted clots in the blood of persons with clotting disorders2, but she and Kell have subsequently found the phenomena in a variety of conditions1, including diabetes, Alzheimer’s illness, and Parkinson’s disease. But, until now, the concept had not acquired any traction.
When the pandemic struck in 2020, Kell and Pretorius applied their methods to individuals sick with SARS-CoV-2 very immediately. “We thought to look at clotting in COVID, because that is what we do,” Pretorius says. Their method employs a unique dye that fluoresces when it attaches to amyloid proteins, such as misfolded fibrin. The glow can then be seen under a microscope by researchers. Plasma samples from 13 healthy participants, 15 people with COVID-19, 10 persons with diabetes, and 11 people with long COVID3 were compared. According to Pretorius, the clotting “was much more than we have previously found in diabetes or any other inflammatory disease” for both long COVID and acute COVID-19. Another study4 looked at the blood of 80 persons with long COVID and discovered micro-clots in every sample.
Only Pretorius, Kell, and their associates have so far published findings about micro-clots in individuals with long COVID.
Caroline Dalton, a neuroscientist at Sheffield Hallam University’s Biomolecular Sciences Research Centre in the United Kingdom, has duplicated the findings in unpublished work. To count the amount of clots in blood, she and her colleagues utilized a somewhat different procedure that involved an automated microscope imaging scanner. The researchers compared three groups of around 25 people: those who had never knowingly had COVID-19, those who had COVID-19 and recovered, and those who had long COVID. All three groups had micro-clots, but those who had never had COVID-19 had less, smaller clots, whereas those who had long COVID had more larger clots. The previously infected group was in the center. The team believes that SARS-CoV-2 infection causes a burst of microclots that eventually disappear. However, they appear to persist in people with long COVID.
Dalton has also discovered that fatigue levels appear to connect with micro-clot counts in a small number of patients. According to Dalton, this “increases confidence that we are measuring something that is mechanistically linked to the condition.”
Long COVID is similar in many aspects to another condition that defies explanation: chronic fatigue syndrome, often known as myalgic encephalomyelitis (ME/CFS). According to Maureen Hanson, director of the US National Institutes of Health (NIH) ME/CFS Collaborative Research Center at Cornell University in Ithaca, New York, Pretorius and Kell’s research has reignited interest in a 1980s-era theory regarding atypical clots contributing to symptoms. Pretorius, Kell, and colleagues discovered amyloid clots in the blood of ME/CFS patients, however the number was substantially lower than in individuals with long COVID5. Pretorius believes that clotting is only a partial explanation for ME/CFS.
Micro-clot mysteries
It is unclear where these micro-clots emerge from. However, Pretorius and Kell believe that the spike protein used by SARS-CoV-2 to enter cells may be the trigger in patients with long COVID. The addition of the spike protein to plasma from healthy individuals in the laboratory was adequate to cause the production of these aberrant clots6.
Evidence suggests that the protein is implicated. Researchers from Harvard University in Boston, Massachusetts, reported identifying the spike protein in the blood of persons with long COVID in a preprint7 published in June. Another paper8, published by a Swedish group, demonstrated that some peptides in the spike can create amyloid strands on their own in a test tube. According to Sofie Nyström, a protein chemist at Linköping University in Sweden and one of the paper’s authors, these misfolded strands could serve as a form of template.
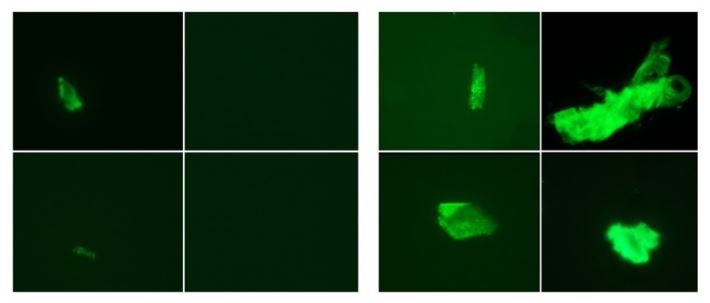
Micro-clots (green) in a study participant before SARS-CoV-2 infection (left four panels) and in the same person after they developed long COVID (right four panels).Credit: E. Pretorius et al./Cardiovasc. Diabetol. (CC BY 4.0)
A team from California discovered that fibrin can adhere to the spike. It was reported in a 2021 preprint9 that when the two proteins bind, fibrin increases inflammation and produces clots that are more difficult to dissolve. But it is unclear how all of these jigsaw pieces fit together.
If the spike protein causes irregular clots, it begs the question of whether COVID-19 vaccinations, which include the spike or instructions for generating it, can also cause them. There is currently no clear proof linking vaccination spikes to clot formation, but Pretorius and Kell have won a grant from the South African Medical Research Council to investigate the matter. (Rare clotting episodes linked to the Oxford-AstraZeneca vaccination are thought to occur via a separate mechanism (read below).)
Raising safety concerns about vaccinations can be difficult, according to Per Hammarström, a protein scientist at Linköping University and one of Nyström’s co-authors. “We don’t want to be over-alarmist, but at the same time, if this is a medical issue, at least in certain people, we have to address that.” Gregory Poland, director of the Mayo Clinic’s vaccination research section in Rochester, Minnesota, feels that the debate is critical. “My guess is that spike and the virus will turn out to have a pretty impressive list of pathophysiologies,” he says. “How much of that may or may not be true for the vaccine, I don’t know.”
Dearth of data
Several experts believe that micro-clots may be a contributing factor to long COVID. And the concept appears to correspond with other clotting data that has surfaced. Researchers are already aware that individuals with COVID-19, particularly those with severe illness, are more likely to produce clots. The virus can infect cells that line the 100,000 kilometers of blood veins in the body, generating inflammation and damage that causes clotting.
These clots may have physiological consequences. Danny Jonigk, a pathologist at Hanover Medical School in Germany, and his colleagues examined tissue samples from COVID-19 victims. They discovered micro-clots and discovered that capillaries had split, generating new branches in an attempt to keep oxygen-rich blood flowing10. The disadvantage was that the branching caused turbulence in the flow, which might lead to new clots.
Several other labs have shown evidence that this clotting tendency continues in some patients months after the initial infection. According to James O’Donnell, a haematologist and clotting specialist at Trinity College Dublin, and his colleagues11, approximately 25% of persons recovering from COVID-19 have symptoms of enhanced clotting that are “quite marked and unusual.”
What is unclear is if this irregular clotting reaction is to account for any of the symptoms of long COVID, or if it is simply “another unusual phenomenon associated with COVID.” According to O’Donnell.
The micro-clot concept, according to Alex Spyropoulos, a haematologist at the Feinstein Institutes for Medical Research in New York City, proposes “a very elegant mechanism.” However, he claims that much more research is needed to link the lab indicators to clinical symptoms. What’s a little bit disturbing is that these authors and others make huge leaps of faith,” Spyropoulos argues.
According to Jeffrey Weitz, a haematologist and clotting expert at McMaster University in Hamilton, Canada, the way Pretorius’ team is utilizing to detect micro-clots “isn’t a standard technique at all.” “I’d like to see confirmation from other investigators,” he adds. Micro-clots are extremely difficult to detect. Pathologists can detect them in tissue samples, but haematologists are more interested in aberrant clotting indicators than in the clots themselves.
Other, larger research of long COVID have not found evidence of clotting. Michael Sneller, an infectious-disease specialist, and his colleagues at the National Institutes of Health in Bethesda, Maryland, evaluated 189 individuals afflicted with SARS-CoV-2, some with lasting signs and some without, as well as 120 controls12. They did not look for micro-clots explicitly. However, if micro-clots were obstructing the capillaries, Sneller believes they should have found indications of tissue damage in capillary-rich organs such as the lungs and kidneys. Micro-clots may potentially harm red blood cells, resulting in anaemia. However, Sneller and his colleagues discovered no evidence of this in any of the lab tests.
Kell and Pretorius believe that just because there was no evidence of micro-clots in this study does not suggest they do not exist. One of the main problems with long COVID is that “every single test comes back within the normal ranges,” according to Pretorius. “You have desperately ill patients with no diagnostic method.” She hopes that other scientists will read their publications and try to reproduce their findings. “Then we can have a discussion,” she says. People with long COVID who feel better after getting anticoagulant medications, she adds, would be the ultimate causative evidence.
There is a small amount of proof for this. In an earlier iteration of a preprint, published in December 2021, Kell, Pretorius, and other investigators noted that 24 individuals with long COVID who were treated with a mixture of two antiplatelet therapies and an anticoagulant experienced some relief13. The researchers’ names were Gert Jacobus Laubscher and others at Stellenbosch University. The main symptoms that the participants were experiencing went away, and they felt less worn out. Additionally, they had less micro-clots. Before attempting to publicly publish these findings, Pretorius and Kell are striving to collect further information. However, other medical professionals are already treating patients with long COVID with these drugs. Some even provide a dialysis-like technique to remove fibrinogen and other inflammatory chemicals from the blood. Such therapy, according to O’Donnell, seems premature. He acknowledges that certain individuals with long COVID are vulnerable to clots, but he believes that extrapolating from a single small study to treating a large number of people “just is not going to wash in 2022 in my book.” Sneller concurs. “Anticoagulating somebody is not a benign thing. You basically are interfering with the blood’s ability to clot,,” he explains, which may render even slight injuries fatal.
Kell is fed up with waiting for an agreement on how to treat long COVID. “These people are in terrible pain. They are desperately unwell, ,” he says. Altmann understands the exasperation. He receives e-mails practically every day asking: “Where are the drug trials? Why does it take so long?” But even in the midst of a pandemic, he argues, researchers have to follow the process. “I’m not rubbishing anybody’s data. I’m just saying we’re not there yet,” he says. “Let’s join up the dots and do this properly.”
- Source : GreatGameIndia